


The Medications Data Interoperability Project (MDIP) was a Horizon funded project conducted by Dynaccurate and Pillcheck to create a large scale database of linked medications sourced from the EU, Canada, UK and USA that flagged medications associated with potential pharmacogenomic impacts.
Our project began in January 2025 and concluded in September 2025. We gathered close to one million medications references from across the target countries and now display those which have a pharmacogenomic flag in a searchable database. Make sure to visit our GitHub if you want to reuse any of the deliverables from this project.
Our objectives
Using AI for Interoperability and Blockchain to combine open source medications data into a user-friendly database.
We use AI technologies to achieve Semantic Interoperability between different medications datasets published or provided by public authorities, such as regulators, terminology severs, medicines agencies or pharma industry bodies.
For each individual source, we write the metadata to the Blockchain as a demonstration of how to ensure provenance.
Highlighting medications that may cause an adverse pharmacogenomic impact, and bring wider attention to this patient safety area.
Pharmacogenomics refers to the branch of genetics which studies the way in which an individual's genetic attributes may affect their personal response to therapeutic drugs. This is also known as a gene/drug reaction, and it can be an adverse effect for a patient, similar to drug/drug and food/drug reactions.
This is an emerging field, but an important one, as gene/drug reactions can be fatal.
An exemplar project of linked open source data demonstrating how new technologies increase trust and reduce costs for authoritative data.
An important part of this project is demonstrating to stakeholders how it is possible to aggregate important, publicly available sources of information. This is necessary to achieve lower spending and wider applications in the field of health, and in this case, the management of medications, stock control, adverse reaction tracking as well supplies and budgetary control. It is also helps fight against counterfeit medications.
Catch up on our latest posts

Video: MDIP final video
Short tutorial video for those who want to use our project deliverables.

Successful Completion of the Medications Data Interoperability Project (MDIP)
Entering our ninth month of the NGI Sargasso MDIP project, we are very happy to announce a successful completion of all tasks.

Video: Maddi details the successful project results from MDIP

Video: Maddi discusses the feasibility of the technologies used in MDIP

Video: Adverse Drug Reactions
Learn more about ADRS in a short video presentation by MADDI.

Adverse Drug Reactions
Check out our new article on Adverse Drug Reactions. They are dangerous, difficult to manage and...

Blockchain for resilience in Healthcare
MADDI discusses resilience in Healthcare data and interoperability. This video demonstrates why we...

Provenance as Security in Healthcare
Why securing cascade systems will be crucial to our future health services. Another article on relating...

Maddi, the MDIP Digital Avatar!
Introducing Maddi - the MDIP Digital Avatar! Our newest member of the team, she will be narrating our future videos!

Progress Update June
I’m particularly proud of how smoothly our MDIP project has been progressing. As of June 2025 we...

Genome Sequencing and Personal Health
Really great video here on genome sequencing here from the Journal of the American Medical Association...

How we establish pharmacogenomic factors related to...
Ruslan is the Chief Scientific Officer of GeneYouIn, the operators of the...

How we are gathering our data
In our execution of the MDIP project, we us a mixture of approaches and tools, both technical and legal. We...

The human costs of non-interoperable data
Check out our latest article on this serious issue here: https://www.linkedin.com/feed/update...
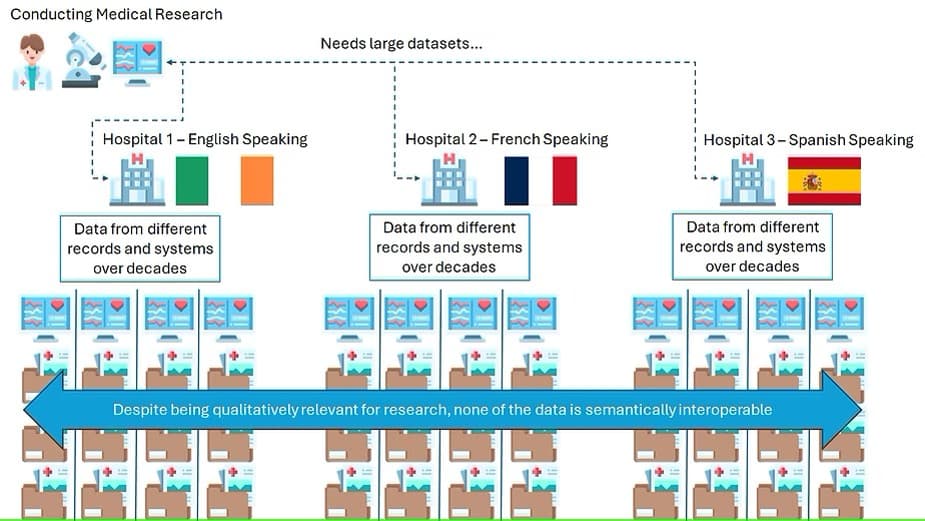
Semantic Interoperability in Health AI
So this article will be a very high level tutorial to...

And the winner is...
The Czech State Institute for Drug Control ( STÁTNÍ ÚSTAV PRO KONTROLU LÉČIV ) for being the ...
New Video!: Semantic Interoperability
Another video in our series. This week, we look at Semantic Interoperability. In order to find medications which may have ...

A little bit more on what we are doing...
If you want to know a little bit more about what we are doing, specifically the technologies in...

Video: What is Pharmacogenomics?
A quick video under 3 minutes to get you up to speed on what is Pharmacogenomics. This is a...

Speaking about MDIP at the Luxembourg European Digital Health Conference 2025
It was a great to be able to introduce our MDIP project and the support we have from NGI...

MDIP Project Website goes live!
www.mdip.eu Delighted to have kicked-off our NGI Sargasso project with our new project...

Dynaccurate and Pillcheck begin work on MDIP project
MDIP: Medications Data Interoperability Project The Medications Data Interoperability...